Abstract
INTRODUCTION
Blinatumomab (BLINA) is a bispecific antibody construct that directs cytolytic T-cells to CD19 expressing B-cells resulting in proliferation of T-cells. It has potent activity in B-cell acute lymphoblastic leukemia (B-ALL) and chronic myeloid leukemia in lymphoid blast phase (CML-BP) and is associated with a risk of central nervous system toxicity (CNS tox) and cytokine release syndrome (CRS). Here we report the incidence, severity, and management of CNS tox and CRS in patients with B-ALL and CML-BP who received BLINA in a real-world setting.
METHODS We retrospectively evaluated pts ≥18 years old with B-ALL or CML-BP treated with BLINA from 2016 to 2020 at MD Anderson Cancer Center. CNS tox and CRS were graded per CTACEv5 (Common Terminology Criteria for Adverse Events). CNS tox excluded headache. The primary objective was to evaluate the incidence of CNS tox and CRS. Secondary objectives included evaluation of toxicity severity, time to toxicity, duration of toxicity, and management of toxicity. Risk factors for toxicity were assessed in a multivariate analysis.
RESULTS
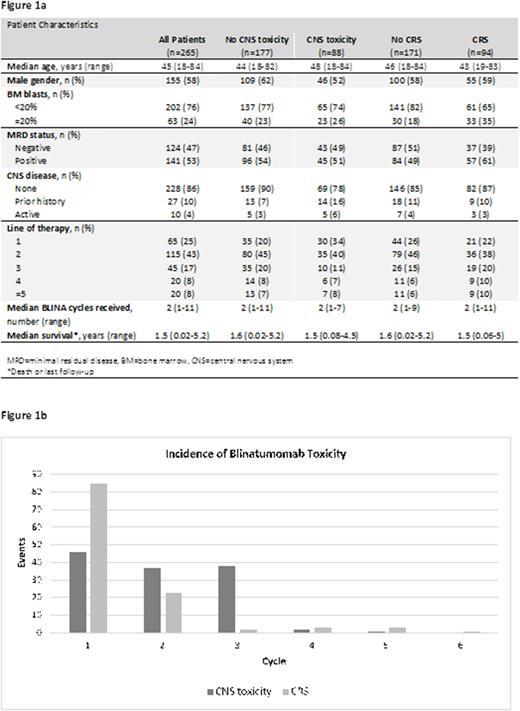
A total of 265 patients (pt) met eligibility criteria. Their median age was 45 years (range, 18-84 years) and 155 (58%) were male (Figures 1A). The cumulative number of BLINA cycles was 754 with a median of 2 cycles per pt (range, 1-11). Sixty-five pts (25%) received BLINA as part of frontline therapy with an overall median number of 1 prior therapy. Prior to starting BLINA, 43 pts (24%) had ≥20% bone marrow blasts and 124 pts (47%) were minimal residual disease (MRD) negative. Ten pts (4%) had active CNS disease before BLINA initiation, 27 pts (10%) had a history of prior CNS disease. A dose ramp-up was performed in 279 BLINA cycles (37%). During these cycles, dose escalation occurred on a median of day 8 (range, 2-17).
Eighty-eight pts (33%) experienced CNS tox while receiving BLINA. CNS tox occurred during 15% (114) of all BLINA cycles and were classified as grade 1, 2, 3, and 4 or greater during 46 (40%), 37 (32%), 28 (25%), and 3 cycles (3%), respectively. Seventy CNS tox cases (61%) occurred during cycle 1, 29 (25%) during cycle 2, and 15 (13%) during cycle 3 or beyond (Figure 1B). Of the CNS tox cases occurring after cycle 1, 23 (52%) had CNS tox during a previous cycle. Median time to onset of CNS tox was 4 days (range, 1-25), median duration of CNS tox was 3 days (range, 1-28). Common symptoms of CNS tox included tremor (n=57), confusion (n=47), weakness (n=11), aphasia (n=9), and gait disturbance (n=8). Of the 114 cycles in which CNS tox occurred, 97 (85%) were managed with steroids. BLINA was held during 71 cycles due to CNS tox (62%) and dose-reduced during 44 cycles (39%). Thirteen cycles with CNS tox (11%) required no intervention. Of the 114 cycles with CNS tox, 66 (58%) were followed by additional cycles of BLINA. Pts who experienced CNS tox received a similar total number of BLINA cycles (median, 2) as pts who did not (median, 2). Pts who experienced CNS tox had a similar median overall survival (1.5 years) to those who did not (1.6 years). Notably, 51% of pts with active CNS disease or a prior history of CNS disease experienced CNS tox compared with 30% of pts without CNS disease. Preliminary multivariate logistic regression identified age, gender, and MRD positivity as risk factors for CNS tox.
Ninety-four pts (35%) experienced CRS. CRS occurred during 115 cycles (15%), of which 36 (31%) were in the setting of a confirmed infection. CRS was classified as grade 1, 2, or 3 during 85 (74%), 23 (20%), and 7 cycles (6%), respectively. Eighty-two CRS cases (71%) occurred during cycle 1, 24 (21%) during cycle 2, and 9 (8%) during cycle 3 or beyond (Figure 1B). Of the CRS cases occurring after cycle 1, 19 (58%) had CRS during a previous cycle. Median onset of CRS was 3 days (range, 1-22), median duration of CRS was 2 days (range, 1-14). CRS was managed with steroids during 83 cycles (72%). BLINA was held during 56 cycles due to CRS (49%) and dose-reduced during 21 cycles (18%). Twenty-six cycles with CRS (23%) required no intervention. Of the 115 cycles with CRS, 79 (69%) were followed by additional cycles of BLINA.
CONCLUSION
CNS tox and CRS occurred in 33% and 35% of pts treated with BLINA and in 15% and 15% of BLINA cycles, respectively. With steroids and therapy modification, most pts who experienced toxicity were able to continue BLINA treatment while exhibiting similar outcomes to those without toxicity.
Disclosures
Jabbour:Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Takeda: Other: Advisory Role, Research Funding. Paul:Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Sasaki:Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka Pharmaceuticals: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees. Short:Stemline Therapeutics: Research Funding; Amgen: Consultancy, Honoraria; AstraZeneca: Consultancy; Pfizer: Consultancy; Takeda Oncology: Consultancy, Research Funding; Astellas: Research Funding; Novartis: Consultancy. Jain:AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; CareDx: Honoraria; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; TG Therapeutics: Honoraria; Cellectis: Honoraria, Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; MEI Pharma: Honoraria; Ipsen: Honoraria; Beigene: Honoraria; TransThera Sciences: Research Funding; Newave: Research Funding; Dialectic Therapeutics: Research Funding; Novalgen: Research Funding; Loxo Oncology: Research Funding; Medisix: Research Funding; Takeda: Research Funding; Mingsight: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Pfizer: Research Funding; Incyte Corporation: Research Funding; Cellectis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; ADC Therapeutics: Research Funding; Servier Pharmaceuticals LLC: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding. Ravandi:Syos: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Prelude: Research Funding; AstraZeneca: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biomea Fusion, Inc.: Research Funding; Amgen: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Amgen: Honoraria, Research Funding. Garcia-Manero:Astex: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; Genentech: Honoraria, Research Funding; Curis: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Gilead Sciences: Research Funding; Novartis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding. Kadia:Glycomimetics: Research Funding; BMS: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; JAZZ: Consultancy, Research Funding; Regeneron: Research Funding; Agios: Consultancy; Novartis: Consultancy; Pfizer: Research Funding; Servier: Consultancy; cellenkos: Research Funding; Ascentage: Research Funding; Genfleet: Research Funding; Astellas: Research Funding; AstraZeneca: Research Funding; Amgen: Research Funding; cyclacel: Research Funding; Delta-Fly: Research Funding; PinotBio: Consultancy; Iterion: Research Funding; Astex: Honoraria; Abbvie: Consultancy, Research Funding. Konopleva:Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding; Forty-Seven; F. Hoffman LaRoche: Honoraria; Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties. Kantarjian:Jazz Pharmaceuticals: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Research Funding; Amgen: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Novartis: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; NOVA Research: Honoraria; Pfizer: Honoraria, Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal